|
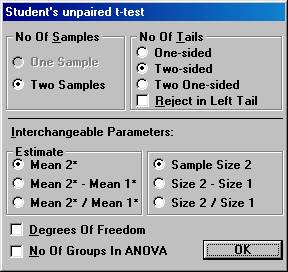
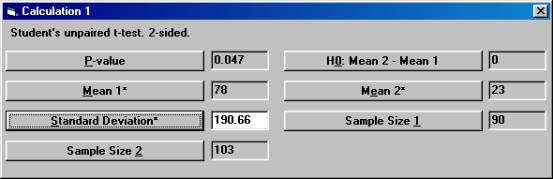
Planning a clinical trial using results from publications. A clinical trial is planned to study the effects of a new drug for treatment of patients with chronic heart failure. Of special interest in this trial is the possible change of neurohormones after 40 weeks of treatment and the plan is to make a comparison with placebo. For ethical reasons, the new drug and placebo will be added to a drug already approved and on the market for the treatment of heart failure. One of the most important neurohormone to study in this trial is aldosterone. To gain some knowledge about what effect would be clinically relevant and what variation one would expect, a literature review was started. 2. Estimation of the variation. Another publication found in the literature review showed a slight significant change in aldosterone for the active drug compared to placebo. The test being used was Student's unpaired t-test and the p-value was 0.047. The number of patients in this study was 90 and 103 in the treatment arm and placebo arm, respectively. The mean change in the treatment group and placebo group was reported to be 78 and 23, respectively. Unfortunately, the standard deviations were reported only at baseline and at the final visit, but not for the change from baseline. To calculate the pooled standard deviation for the change in aldosterone used in the t-test, Open the File menu and choose New Calculation. Open the Test Procedures menu and choose Student's unpaired t-test Choose the option button Two-sided and among the Interchangeable Parameters options Mean 2* and Sample Size 2 and press OK.
Set the parameter values except for p-value as shown, and then press the Standard Deviation* button.
The textbox for Standard Deviation* will then show the value 190.66, the standard deviation that was used but not reported in the publication. |